Scientist Proposes New Method to Identify Toxic Disinfection Byproducts in Drinking Water
By Dan DeBaunShare
Consumption of treated drinking water puts us at risk of exposure to a wide concoction of organic chemical compounds — both natural and man-made — which are formed during the water treatment process. These include disinfection byproducts that typically form as a result of chemical reactions that occur when disinfection chemicals, such as chlorine, react with other chemicals or substances present in water. Since the contaminant mixtures that arise as a result of the treatment process tend to be rather complex, assessing the health impacts of exposure to these contaminants has proven difficult.
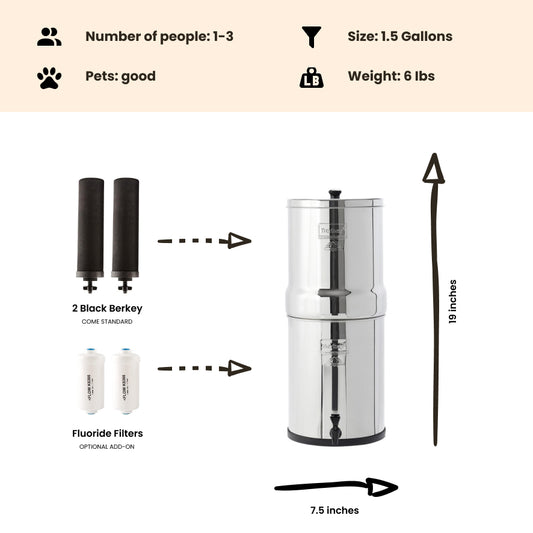
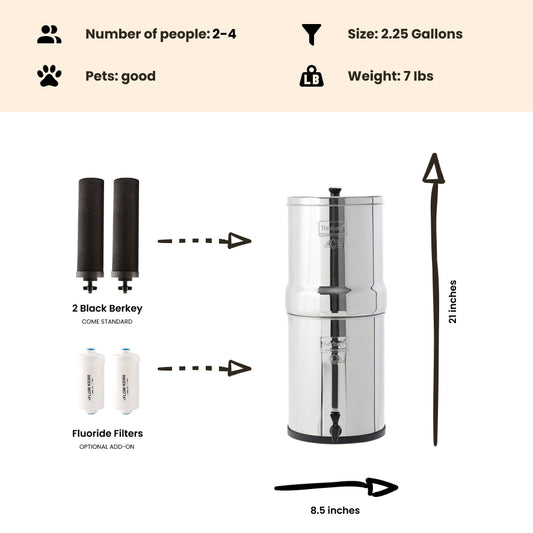
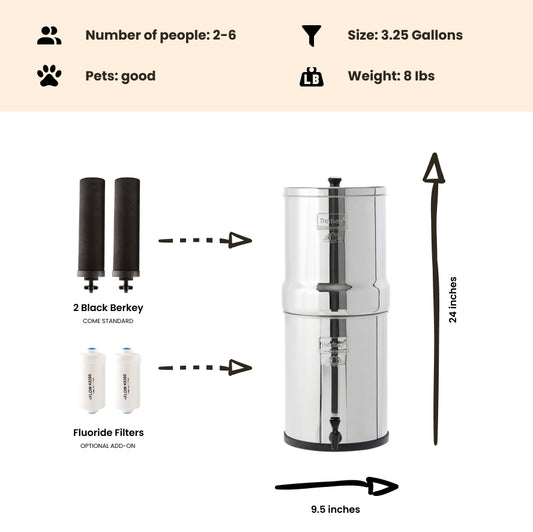
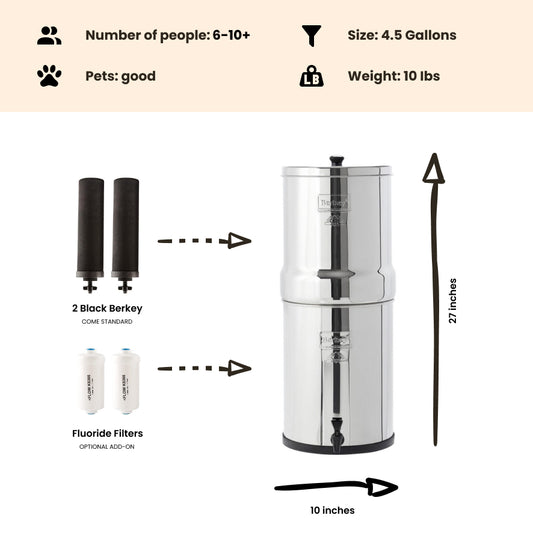
Note: The Berkey water filter systems equipped with the standard black berkey filters WILL remove THM (Trihalomethanes) / disinfection byproducts from the water to below lab detectable limits!
Trihalomethanes, or THMs as they commonly referred to, are four chemicals that together with other types of disinfection byproducts form as a result of a chemical reaction that occurs between chlorine (or similar disinfectants that are added to drinking water to kill harmful microbes) and organic and inorganic material that may be present in the water.
Yet while consumers tend to be aware that disinfection chemicals are added to their drinking water in the treatment process to kill off any harmful pathogens that may be present, they may be unaware that the use of these chemicals can result in the formation of toxic byproducts. It is also concerning that very little is known about the health impact of these chemicals, which are unregulated and therefore unmonitored.

In a research paper which was recently published in Environmental Science: Processes & Impacts, Carsten Prasse, a professor in the Department of Environmental Health and Engineering at John Hopkins University, proposes a new approach for assessing the quality of drinking water, which could vastly improve the quality of water flowing from our taps.
"We are exposing people in the United States to these chemical compounds without knowing what they even do," Prasse said. "I'm not saying that chlorination is not important in keeping our drinking water safe. But there are unintended consequences that we have to address and that the public needs to know about. We could do more than what we're doing."
It is concerning that only eleven compounds out of more than 700 disinfection byproducts that have been identified in chlorinated water to date, are currently regulated.
According to Prasse, these eleven compounds have been regulated since the 1990s, but no additional disinfection byproducts have been added to the watchlist despite their being strong scientific evidence that other toxic disinfection byproducts are present in drinking water. Existing methods used to evaluate the health impacts of chemical compounds in drinking water are not only outdated, but also extremely tedious, and usually rely on animal studies that are both time consuming and expensive to conduct.
Applying these methods to the ever increasing list of disinfection chemicals present in drinking water would not be feasible from an economic standpoint, Prasse explains, adding that at the very minimum, new methods are required to identify chemical compounds that pose the greatest risk.
To do this, Prasse proposes that we need to cast a bigger net if we hope to identify a wider range of chemical compounds present in water samples collected. According to Prasse, the "reactivity-directed analysis" is able to provide a much broader assessment of what chemicals are present in water as it targets the largest group of chemical toxins known as "organic electrophiles".
"This method can help us prioritize which chemicals we need to be paying closer attention to with possible new regulations and new limits while saving time and resources," Prasse said.
Prasse's proposed approach utilizes recent advances in molecular toxicology and analytical chemistry to identify chemical toxins according to how they react with biomolecules in the bodies of living organisms that are essential for biological process, such as amino acids which serve as protein building blocks, for example. By simulating this process, this new approach is able to identify chemical toxins in drinking water.
"We know that the toxicity of many chemicals is caused by their reaction with proteins or DNA which alter their function and can result, for example, in cancer," Prasse said.
Journal Reference
Carsten Prasse. Reactivity-directed analysis – a novel approach for the identification of toxic organic electrophiles in drinking water. Environ. Sci.: Processes Impacts, (2021),23, 48-65; doi:10.1039/D0EM00471E
-
Regular price $234.00 USDRegular priceUnit price / per
-
Regular price $327.00 USDRegular priceUnit price / per
-
Regular price From $367.00 USDRegular priceUnit price / per
-
Regular price From $408.00 USDRegular priceUnit price / per
-
Regular price From $451.00 USDRegular priceUnit price / per
-
Regular price From $478.00 USDRegular priceUnit price / per
-
Regular price $332.50 USDRegular priceUnit price / per
$350.00 USDSale price $332.50 USDSale

Dan DeBaun is the owner and operator of Big Berkey Water Filters. Prior to Berkey, Dan was an asset manager for a major telecommunications company. He graduated from Rutgers with an undergraduate degree in industrial engineering, followed by an MBA in finance from Rutgers as well. Dan enjoys biohacking, exercising, meditation, beach life, and spending time with family and friends.
~ The Owner of Big Berkey Water Filters